How to Outsmart Aging Cheaply: A Practical Guide to the Science of Staying Younger—Affordably
I mapped the molecular causes of aging—and found low-cost, high-impact interventions for each one.
What the heck is NAD+ and why do people get it injected?
Why the sudden craze in cold plunges when it used to be reserved for the Finnish?
When did starving yourself get a hip rebrand to fasting?
This decade has seen a dramatic uptick in longevity science, evident not just in the explosion of the longevity biotech industry (projected to reach $600 billion by 2028), but also with the very intelligent individuals (not just biohacker bros) confidently proclaiming that death will soon, be something we can delay…indefinitely?
Sure, some of these individuals are biased because their career lies in the success of extending lifespan (i.e., Harvard geneticist David Sinclair), or because they invested half of their life savings into the cause (a modest $180 million by Sam Altman).
But people without direct monetary ties are also bullish on living longer, including Dario Amodei, Princeton Physicist and founder of Anthropic, who in his essay about the benefits of AI on society, somewhat casually noted that we may be able to buy ourselves “enough time that most of those currently alive today will be able to live as long as they want.”
Yet as often occurs in the cycle of innovation, there is a large knowledge gap between scientific research and general public knowledge.
On one hand, there are 700+ biotech companies racing to be the first company to make an FDA- approved longevity treatment, and their amazing research often sounds right out of a sci-fi movie.
Some sci-fi-esque highlights include:
Fauna Bio, which in a gross simplification, looks at the science of mammalian hibernation to see if similar processes can be applied to humans
Insilico Medicine, which already has its first AI-discovered drug in Phase 2 trials (if you’re shocked that AI can do that, I highly recommend you read Dario Amodei’s article I referenced above)
On the other hand, many of us are still stuck wondering whether those weird ingredients on the back of the labels of our protein bars really will kill us (p.s. avoid them when you can, but they probably won’t kill you).
And the vast majority of us? Us being the American population?
We have over a 40% chance of being obese and, in conservative estimates, a ~50% chance of dying from something preventable. Yikes.
Add to the the politicization of health in the USA, and the brewing rage at Big Health exemplified—quite brutally—by Luigi Mangione’s manifesto, and let’s say that from a systems perspective of health, we are living in a complicated world.
Yikes.
Fortunately, if you move outside of the noise, you’ll find that a lot of the science-backed basics of longevity health are:
Quite simple (although simple does not mean easy…I’ve done a 36 hour fast a total of one time)
Affordable (for the most part — I acknowledge the price of supplements can add up)
Overlap with each other
Below, I overview the pathways of aging that have simple and affordable protocols to help you stop, or in some cases reverse, aging in its track.
However, before scrolling below, please note a few important caveats:
There is no scientific consensus on the specific hallmarks of aging, and the list of emerging hallmarks has been growing since Lopez-Otin et al’s (2013) publication.
The hallmarks of aging framework has received much criticism because it leads to a reductionist approach, so while you can map most of these pathways to hallmarks, they are not a perfect 1:1.
While each intervention has scientific evidence, the rigor varies, given this is a relatively new field.
Okay, enough of the jargony science, lets get to the fun stuff.
Enjoy!
1. Genomic Instability
What it is: Our DNA accumulates damage over time from sources like radiation, toxins, and replication errors. The resulting DNA mutations, breaks, and chromosomal aberrations contributes to aging by impairing cell function and raising cancer risk.
Why it matters: Methods to preserve DNA integrity and enhancing repair mechanisms can cumulatively, over a lifetime slow down DNA damage and therefore support healthier aging.
2. Telomere Attrition
What it is: Telomeres are the protective caps on chromosome ends. Each cell division shortens telomeres, and when they get too short, the cell malfunctions or stops dividing (enter senescence).
Why it matters: Telomeres essentially measure a cell’s “biological age.” Interventions that slow telomere loss can keep cells dividing healthily (this is important, you don’t want uncontrolled cell growth) for longer. Chronic stress, for example, is notorious for hastening telomere shortening. Slowing telomere attrition, or in the best case scenario, lengthening telomeres, helps maintain the regenerative capacity of cells.
3. Epigenetic Alterations
What it is: If our genes were hardware, epigenetics would be the software telling genes when and how to work. As we age, this software gets buggy and critical repair genes (e.g. DNA repair, anti-inflammatory) might get turned off, while harmful ones get turned on.
Why it matters: Fortunately, epigenetic marks are malleable, meaning lifestyle and diet can positively influence them – effectively slowing our “epigenetic clocks” and keeping our genetic software “younger.” For instance, research shows that exercise and healthier diets have slowed the rate at which DNA methylation patterns age by over 3 years in as little as 8 weeks!
4. Loss of Proteostasis
What it is: Proteins are the molecular machines of our cells: when they misfold or break, cells start to malfunction. Proteostasis means maintaining the quality and balance of all proteins in the body. Our cells constantly fold, refold, and dispose of proteins. With age, this quality control falters – misfolded and aggregated proteins build up (e.g., Alzheimer’s plaques or cataracts).
Why it matters: Enhancing the cleanup of proteins, such as via excercise, can delay aging. Cleaning up proteins includes anything that ramps up autophagy, our cellular recycling center. Autophagy is so critical (imagine what would happen to your home if you never threw away your trash…) to other hallmarks of aging that it is often considered the overarching center of aging pathways. Keeping your proteins properly folded and disposed means cells remain functional and resilient longer.
5. Deregulated Nutrient Sensing
What it is: Our cells sense nutrients and adjust growth or repair pathways accordingly. Aging is marked by deregulated nutrient sensing: i.e., metabolic signals going haywire. Key pathways include insulin/IGF-1 (carb/protein sensing), mTOR (proteins), AMPK (low energy), and sirtuins (NAD⁺/low energy). In youth, these signals balance growth and maintenance. With age (and overeating), constant nutrient signals (high insulin, IGF-1, mTOR) accelerate aging, while nutrient-scarcity signals (AMPK, sirtuins) promote longevity.
Why it matters: This hallmark is essentially about avoiding “too much fuel” signals, as happens with high calorie or high protein diets. When nutrient sensors like insulin and mTOR are constantly high, cells prioritize growth and reproduction at the expense of maintenance and repair. Over time, that accelerates aging.
You can think of it as revving an engine non-stop. In contrast, dialing down those signals puts cells in a stress-resistant self-repair mode. That is why caloric restriction tends to extend lifespan in animal studies.
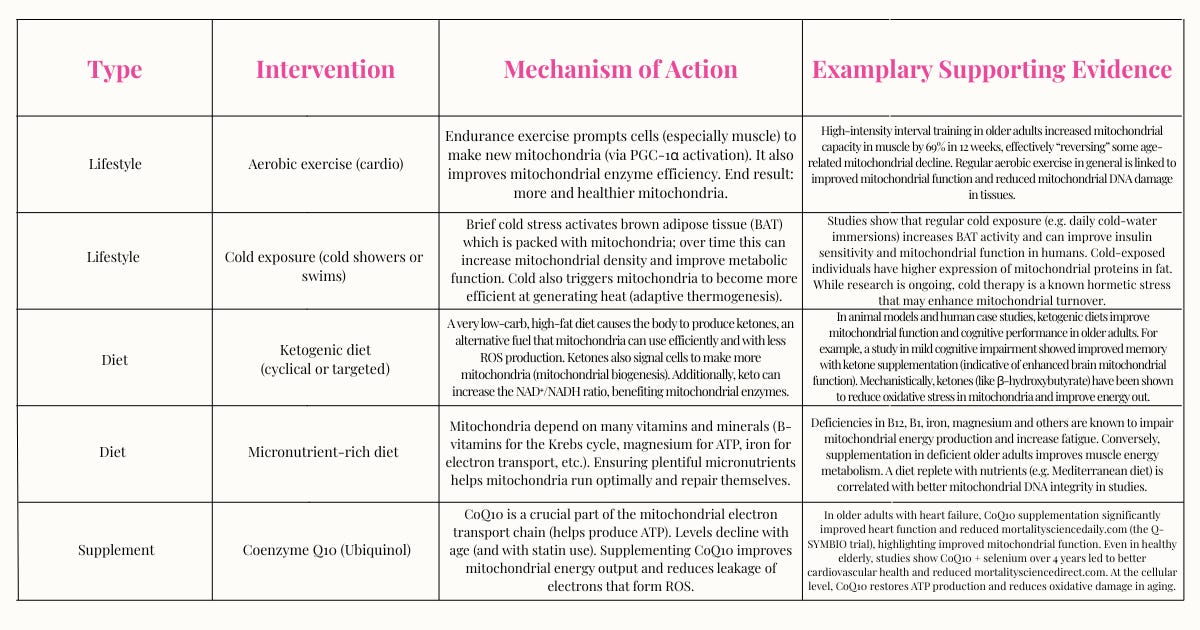
6. Mitochondrial Dysfunction
What it is: Mitochondria are the cell’s power plants (remember the Krebs Cycle from high school biology class?). They produce energy (ATP) but also generate reactive oxygen species (ROS) as a byproduct. With age, mitochondria dysfunction, becoming less efficient and more damaged, leading to lower energy, more cell death, and inflammation. By midlife, many people’s cells are not producing energy like they used to, which explains why you had more energy in your 20’s than you do now.
Why it matters: Improving mitochondrial health can increase energy levels and reduce aging. Key strategies include stimulating mitochondrial biogenesis (making new mitochondria), enhancing mitochondrial efficiency, and reducing mitochondrial damage from ROS.
7. Cellular Senescence
What it is: Senescent cells, sometimes called “zombie cells” are older or damaged cells that have permanently stopped dividing but don’t die off. Like one bad apple rotting the bunch, they secrete harmful inflammatory molecules that then damage neighboring cells. Clearing them out or preventing their accumulation is a promising anti-aging strategy.
Why it matters: Reducing the formation of or actively removing (senolysis) senescent cells is one of the most exciting frontiers in anti-aging research because it directly reverses damage: for example, old mice given senolytics became measurably younger, with stronger hearts, faster running, and even longer median lifespan!
8. Stem Cell Exhaustion
What it is: Stem cells are the body’s reservoir of new cells – they replace cells that die or are damaged. With age, stem cells get exhausted or go dormant. For example, older people have fewer functioning blood stem cells, muscle stem cells, etc., leading to poorer healing, weaker immune response, and tissue atrophy.
Why it matters: Stem cells are like the “fountain of youth” inside us—preserving stem cell quantity and function is critical to maintain organ regeneration. The goal is to protect them from damage (so they don’t die or senesce), and stimulate them to stay active (so they continue replenishing tissues). While chronic inflammation or deficiency tells stem cells “it’s dangerous or pointless to divide,” nutritional and lifestyle interventions may give stem cells the support to do their job.
9. Altered Intercellular Communication
What it is: Every cell in your body is constantly delivering messages that affect its neighbors and even far-away organs (via hormones, neurotransmitters, cytokines, etc.). Aging cranks up the volume on “bad” messages – like chronic inflammatory cytokines –which can accelerate all other hallmarks (genomic instability, senescence, etc.).
Why it matters: As we age, the way our cells communicate with each other become deregulated. This includes an increase in pro-aging signals like chronic inflammation (“inflammaging”, a condition so impactful I go over it on its own below), and a decline in youthful signals (e.g. growth factors, sex hormones). Interventions aim to restore a healthier communication environment, reducing noise from inflammatory signals and boosting positive signals.
10. Chronic Inflammation (Inflammaging)
What it is: One of the newly recognized “hallmarks,” inflammaging is the persistent, low-grade inflammation that develops with age. It’s driven by any factor that puts your body in “alert” mode (senescent cells, visceral fat, gut microbiome changes, etc.) and contributes to tissue damage, insulin resistance, and most age-related diseases.
Why it matters: Chronic inflammation over time harms healthy cells and exhausts stem cells and the immune system. By dousing it, you create a more youthful internal environment. Many interventions converge on one theme: reduce pro-inflammatory triggers (like visceral fat, smoking, high sugar) and increase anti-inflammatory inputs (like exercise, omega-3, fiber). The payoff is multifold: you’ll likely feel better (less achy, more energetic) and your risk of diseases drops.
11. Gut Dysbiosis
What it is: The gut microbiome, the trillions of bacteria in our intestines, plays a major role in aging. With age (and with bad diets or medications), beneficial microbes decrease, and harmful ones increase (dysbiosis, another new hallmark of aging). This ties into altered nutrient signaling. gut permeability (“leaky gut”), the release of endotoxins that drive inflammation (linking to inflammaging),and even
Why it matters: Your gut microbiome is like a control center that interfaces with your immune, metabolic, and nervous systems. Restoring your microbiome helps reduce inflammation, improve nutrient sensing, and strengthen immunity. Over time, you might notice improvements like better digestion, stronger immunity (fewer infections), and even better mood and thinking (since gut microbes produce mood-enhancing neurotransmitters and modulators like GABA and serotonin)!
12. Extracellular Matrix (ECM) Remodeling Dysfunction
What it is: The extracellular matrix, an emerging hallmark of aging, is the structural network of proteins (like collagen and elastin) outside our cells. Quite literally, the state of your ECM is how well your body holds together. With age, the ECM becomes stiffer and less elastic due to cross-linking of fibers (often from sugar-driven glycation) and reduced turnover of old proteins.
Why it matters: Youthful ECM means flexible arteries, resilient joints and tendons, and smoother, firmer skin. Therefore, when ECM degrades, in addition to its effects on your skin, it also contributes to hypertension, heart disease, kidney disease, and frailty from weak connective tissue. Fortunately, it’s never too late to improve: studies show even in one’s 60s and 70s, following these practices can improve ECM!
If you’ve made it this far, you’ve probably noticed some common themes:
A variety of movement (not just one type of exercise)
A diet rich in micronutrients, low in added sugars and processed junk
A few targeted supplements (if your budget allows)
Some kind of mind-body or stress-reduction practice
And yes, sigh, a bit of fasting…if it works for you
In short? Nothing that feels like a full time job or requires a six-figure salary.
I know it’s a lot to take in. But knowledge is power — and this is just the start.
I’ll be breaking down each aging pathway (and the low-cost protocols to improve them) in upcoming posts.
So stick around, I’m just getting started.
Hi, I’m Arianna—founder of a plant-powered superfoods company and longtime wellness nerd. With 3 full-scholarship Ivy League degrees and a PhD in org, behavior, I’ve spent the last decade helping people that are either too busy or too budget-restrained feel better, think sharper, and age slower.. Subscribe for your weekly dose of science-backed longevity.
REFERENCES
Horvath, S. (2013). DNA methylation age of human tissues and cell types. Genome Biology, 14(10), R115. https://doi.org/10.1186/gb-2013-14-10-r115
Kennedy, B. K., Berger, S. L., Brunet, A., Campisi, J., Cuervo, A. M., Epel, E. S., ... & Sierra, F. (2014). Geroscience: Linking aging to chronic disease. Cell, 159(4), 709–713. https://doi.org/10.1016/j.cell.2014.10.039
Kirkwood, T. B. L. (2005). Understanding the odd science of aging. Cell, 120(4), 437–447. https://doi.org/10.1016/j.cell.2005.01.027
Levine, M. E., Lu, A. T., Quach, A., Chen, B. H., Assimes, T. L., Bandinelli, S., ... & Horvath, S. (2018). An epigenetic biomarker of aging for lifespan and healthspan. Aging, 10(4), 573–591. https://doi.org/10.18632/aging.101414
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M., & Kroemer, G. (2013). The hallmarks of aging. Cell, 153(6), 1194–1217. https://doi.org/10.1016/j.cell.2013.05.039
GENOMIC INSTABILITY
Burhans, W. C., & Weinberger, M. (2007). DNA replication stress, genome instability and aging. Nucleic Acids Research, 35(22), 7545–7556. https://doi.org/10.1093/nar/gkm1059
Fenech, M. (2001). The role of folic acid and vitamin B12 in genomic stability of human cells. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 475(1–2), 57–67. https://doi.org/10.1016/S0027-5107(01)00073-1
Gomes, A. P., Price, N. L., Ling, A. J. Y., Moslehi, J. J., Montgomery, M. K., Rajman, L., ... & Sinclair, D. A. (2013). Declining NAD+ induces a pseudohypoxic state disrupting nuclear–mitochondrial communication during aging. Cell, 155(7), 1624–1638. https://doi.org/10.1016/j.cell.2013.11.037
Li, Y., He, X., & Li, C. (2020). Curcumin protects against oxidative stress-induced DNA damage and apoptosis in human umbilical vein endothelial cells through the Nrf2 pathway. Pharmaceutical Biology, 58(1), 479–486. https://doi.org/10.1080/13880209.2020.1761623
Packer, L., & Cadenas, E. (2007). Lipoic acid: Energy metabolism and redox regulation of transcription and cell signaling. Journal of Clinical Biochemistry and Nutrition, 40(1), 1–5. https://doi.org/10.3164/jcbn.40.1
Ristow, M., & Schmeisser, K. (2014). Mitohormesis: Promoting health and lifespan by increased levels of reactive oxygen species (ROS). Dose-Response, 12(2), 288–341. https://doi.org/10.2203/dose-response.13-035.Ristow
Vaziri, H., & Benchimol, S. (1998). Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative lifespan. Current Biology, 8(5), 279–282. https://doi.org/10.1016/S0960-9822(98)70113-5
Zwart, S. R., Gibson, C. R., Mader, T. H., Ericson, K., Ploutz-Snyder, R., Heer, M., ... & Smith, S. M. (2012). Vision changes after spaceflight are related to altered folate- and vitamin B-12-dependent one-carbon metabolism. The Journal of Nutrition, 142(3), 427–431. https://doi.org/10.3945/jn.111.154245
TELOMERE ATTRITION
Blackburn, E. H., Epel, E. S., & Lin, J. (2015). Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science, 350(6265), 1193–1198. https://doi.org/10.1126/science.aab3389
Boccardi, V., & Boccardi, M. (2019). Telomerase activation: A potential key modulator for human healthspan and longevity. Ageing Research Reviews, 55, 100963. https://doi.org/10.1016/j.arr.2019.100963
Carlson, L. E., Beattie, T. L., Giese‐Davis, J., Faris, P., Tamagawa, R., Fick, L. J., ... & Speca, M. (2015). Mindfulness-based cancer recovery and supportive-expressive therapy maintain telomere length relative to controls in distressed breast cancer survivors. Cancer, 121(3), 476–484. https://doi.org/10.1002/cncr.29063
Kiecolt-Glaser, J. K., Epel, E. S., Belury, M. A., Andridge, R., Lin, J., Glaser, R., ... & Malarkey, W. B. (2013). Omega-3 fatty acids, oxidative stress, and leukocyte telomere length: A randomized controlled trial. Brain, Behavior, and Immunity, 28, 16–24. https://doi.org/10.1016/j.bbi.2012.09.009
Ornish, D., Lin, J., Chan, J. M., Epel, E., Kemp, C., Weidner, G., ... & Blackburn, E. H. (2013). Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. The Lancet Oncology, 14(11), 1112–1120. https://doi.org/10.1016/S1470-2045(13)70366-8
Puterman, E., Lin, J., Blackburn, E. H., O'Donovan, A., Adler, N. E., & Epel, E. S. (2010). The power of exercise: Buffering the effect of chronic stress on telomere length. PLoS ONE, 5(5), e10837. https://doi.org/10.1371/journal.pone.0010837
EPIGENETIC ALTERATIONS
Bahar, R., Hartmann, C. H., Rodriguez, K. A., Denny, A. D., Busuttil, R. A., Dolle, M. E. T., ... & Vijg, J. (2006). Increased cell-to-cell variation in gene expression in ageing mouse heart. Nature, 441(7096), 1011–1014. https://doi.org/10.1038/nature04844
Fahy, G. M., Brooke, R. T., Watson, J. P., Good, Z., Vasanawala, S. S., Maecker, H., ... & Horvath, S. (2019). Reversal of epigenetic aging and immunosenescent trends in humans. Aging Cell, 18(6), e13028. https://doi.org/10.1111/acel.13028
Li, Y., Daniel, M., & Tollefsbol, T. O. (2011). Epigenetic regulation of caloric restriction in aging. BMC Medicine, 9(1), 98. https://doi.org/10.1186/1741-7015-9-98
McGee, S. L., & Walder, K. R. (2017). Exercise and the skeletal muscle epigenome. Cold Spring Harbor Perspectives in Medicine, 7(9), a029876. https://doi.org/10.1101/cshperspect.a029876
Pal, S., & Tyler, J. K. (2016). Epigenetics and aging. Science Advances, 2(7), e1600584. https://doi.org/10.1126/sciadv.1600584
Wang, Y., Surzenko, N., Friday, W. B., & Kageyama, R. (2020). Intermittent fasting preserves epigenetic youth and promotes survival. Nature Communications, 11, 3205. https://doi.org/10.1038/s41467-020-16876-0
LOSS OF PROTEOSTASIS
Cohen, H. Y., Miller, C., Bitterman, K. J., Wall, N. R., Hekking, B., Kessler, B., ... & Sinclair, D. A. (2004). Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science, 305(5682), 390–392. https://doi.org/10.1126/science.1099196
Hipp, M. S., Kasturi, P., & Hartl, F. U. (2019). The proteostasis network and its decline in ageing. Nature Reviews Molecular Cell Biology, 20(7), 421–435. https://doi.org/10.1038/s41580-019-0101-y
Labbadia, J., & Morimoto, R. I. (2015). The biology of proteostasis in aging and disease. Annual Review of Biochemistry, 84, 435–464. https://doi.org/10.1146/annurev-biochem-060614-033955
Madeo, F., Zimmermann, A., Maiuri, M. C., & Kroemer, G. (2015). Essential role for autophagy in life span extension. The Journal of Clinical Investigation, 125(1), 85–93. https://doi.org/10.1172/JCI73946
Powers, E. T., Morimoto, R. I., Dillin, A., Kelly, J. W., & Balch, W. E. (2009). Biological and chemical approaches to diseases of proteostasis deficiency. Annual Review of Biochemistry, 78, 959–991. https://doi.org/10.1146/annurev.biochem.052308.114844
DEREGULATED NUTRIENT SENSING
Barzilai, N., Crandall, J. P., Kritchevsky, S. B., & Espeland, M. A. (2016). Metformin as a tool to target aging. Cell Metabolism, 23(6), 1060–1065. https://doi.org/10.1016/j.cmet.2016.05.011
Baur, J. A., & Sinclair, D. A. (2006). Therapeutic potential of resveratrol: The in vivo evidence. Nature Reviews Drug Discovery, 5(6), 493–506. https://doi.org/10.1038/nrd2060
Fontana, L., & Partridge, L. (2015). Promoting health and longevity through diet: From model organisms to humans. Cell, 161(1), 106–118. https://doi.org/10.1016/j.cell.2015.02.020
Longo, V. D., & Panda, S. (2016). Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metabolism, 23(6), 1048–1059. https://doi.org/10.1016/j.cmet.2016.06.001
Madeo, F., Pietrocola, F., & Kroemer, G. (2014). Caloric restriction mimetics: Towards a molecular definition. Nature Reviews Drug Discovery, 13(10), 727–740. https://doi.org/10.1038/nrd4391
Weir, H. J., Yao, P., Huynh, F. K., Escoubas, C. C., Goncalves, R. L. S., Burkewitz, K., ... & Mair, W. B. (2017). Dietary restriction and AMPK increase lifespan via mitochondrial network and peroxisome remodeling. Cell Metabolism, 26(6), 884–896.e5. https://doi.org/10.1016/j.cmet.2017.09.024
MITOCHONDRIAL DYSFUNCTION
Andreux, P. A., Houtkooper, R. H., & Auwerx, J. (2013). Pharmacological approaches to restore mitochondrial function. Nature Reviews Drug Discovery, 12(6), 465–483. https://doi.org/10.1038/nrd4025
Chung, J. H., Seo, A. Y., Chung, S. W., Kim, M. K., Leeuwenburgh, C., & Yu, B. P. (2009). Mitochondrial aging and caloric restriction. Aging Cell, 8(2), 125–138. https://doi.org/10.1111/j.1474-9726.2009.00460.x
Gonzalez-Freire, M., de Cabo, R., Studenski, S. A., & Ferrucci, L. (2015). The neuromuscular junction: Aging at the crossroad between nerves and muscle. Frontiers in Aging Neuroscience, 7, 208. https://doi.org/10.3389/fnagi.2015.00208
Kelley, D. E., He, J., Menshikova, E. V., & Ritov, V. B. (2002). Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes, 51(10), 2944–2950. https://doi.org/10.2337/diabetes.51.10.2944
Short, K. R., Bigelow, M. L., Kahl, J., Singh, R., Coenen-Schimke, J., Raghavakaimal, S., & Nair, K. S. (2005). Decline in skeletal muscle mitochondrial function with aging in humans. Proceedings of the National Academy of Sciences, 102(15), 5618–5623. https://doi.org/10.1073/pnas.0501559102
CELLULAR SENESCENCE
Baker, D. J., Wijshake, T., Tchkonia, T., LeBrasseur, N. K., Childs, B. G., van de Sluis, B., ... & van Deursen, J. M. (2011). Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature, 479(7372), 232–236. https://doi.org/10.1038/nature10600
Justice, J. N., Nambiar, A. M., Tchkonia, T., LeBrasseur, N. K., Pascual, R., Hashmi, S. K., ... & Kirkland, J. L. (2019). Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine, 40, 554–563. https://doi.org/10.1016/j.ebiom.2018.12.052
Xu, M., Pirtskhalava, T., Farr, J. N., Weigand, B. M., Palmer, A. K., Weivoda, M. M., ... & Kirkland, J. L. (2018). Senolytics improve physical function and increase lifespan in old age. Nature Medicine, 24(8), 1246–1256. https://doi.org/10.1038/s41591-018-0092-9
Zhu, Y., Tchkonia, T., Pirtskhalava, T., Gower, A. C., Ding, H., Giorgadze, N., ... & Kirkland, J. L. (2015). The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell, 14(4), 644–658. https://doi.org/10.1111/acel.12344
STEM CELL EXHAUSTION
Chambers, S. M., Shaw, C. A., Gatza, C., Fisk, C. J., Donehower, L. A., & Goodell, M. A. (2007). Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biology, 5(8), e201. https://doi.org/10.1371/journal.pbio.0050201
Rossi, D. J., Jamieson, C. H., & Weissman, I. L. (2008). Stems cells and the pathways to aging and cancer. Cell, 132(4), 681–696. https://doi.org/10.1016/j.cell.2008.01.036
Yousefzadeh, M. J., Schafer, M. J., Noren Hooten, N., Atkinson, E. J., Evans, M. K., Baker, D. J., ... & Niedernhofer, L. J. (2021). Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine, 36, 18–28. https://doi.org/10.1016/j.ebiom.2018.09.015
ALTERED INTERCELLULAR COMMUNICATION
Franceschi, C., Garagnani, P., Parini, P., Giuliani, C., & Santoro, A. (2018). Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nature Reviews Endocrinology, 14(10), 576–590. https://doi.org/10.1038/s41574-018-0059-4
Zhang, G., Li, J., Purkayastha, S., Tang, Y., Zhang, H., Yin, Y., ... & Cai, D. (2013). Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature, 497(7448), 211–216. https://doi.org/10.1038/nature12143
Zhu, Y., Tchkonia, T., Fuhrmann-Stroissnigg, H., Dai, H. M., Ling, Y. Y., Stout, M. B., ... & Kirkland, J. L. (2016). Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell, 15(3), 428–435. https://doi.org/10.1111/acel.12445
CHRONIC INFLAMMATION
Ferrucci, L., & Fabbri, E. (2018). Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nature Reviews Cardiology, 15(9), 505–522. https://doi.org/10.1038/s41569-018-0064-2
Franceschi, C., Garagnani, P., Parini, P., Giuliani, C., & Santoro, A. (2018). Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nature Reviews Endocrinology, 14(10), 576–590. https://doi.org/10.1038/s41574-018-0059-4
Zhou, X., Jiang, X., & Wang, Y. (2023). Inflammation and aging: Signaling pathways and intervention strategies. Signal Transduction and Targeted Therapy, 8(1), 1–20. https://doi.org/10.1038/s41392-023-01502-8Nature
Fulop, T., Larbi, A., Pawelec, G., Khalil, A., Cohen, A. A., Hirokawa, K., ... & Franceschi, C. (2021). Immunology of aging: The birth of inflammaging. Clinical Reviews in Allergy & Immunology, 61(1), 1–14. https://doi.org/10.1007/s12016-020-08853-1Wikipedia
Dugan, B., Conway, J., & Duggal, N. A. (2023). Inflammaging as a target for healthy ageing. Age and Ageing, 52(2), afac328. https://doi.org/10.1093/ageing/afac328Wikipedia
Frasca, D., & Blomberg, B. B. (2016). Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology, 17(1), 7–19. https://doi.org/10.1007/s10522-015-9578-8Wikipedia
Franceschi, C., Bonafè, M., & Valensin, S. (2000). Inflamm-aging: An evolutionary perspective on immunosenescence. Annals of the New York Academy of Sciences, 908(1), 244–254. https://doi.org/10.1111/j.1749-6632.2000.tb06651.xWikipedia
Zhu, Y., Armstrong, J. L., Tchkonia, T., & Kirkland, J. L. (2014). Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Current Opinion in Clinical Nutrition & Metabolic Care, 17(4), 324–328. https://doi.org/10.1097/MCO.0000000000000065
GUT DYSBIOSIS
O'Toole, P. W., & Jeffery, I. B. (2015). Gut microbiota and aging. Science, 350(6265), 1214–1215. https://doi.org/10.1126/science.aac8469
Claesson, M. J., Jeffery, I. B., Conde, S., Power, S. E., O'Connor, E. M., Cusack, S., ... & O'Toole, P. W. (2012). Gut microbiota composition correlates with diet and health in the elderly. Nature, 488(7410), 178–184. https://doi.org/10.1038/nature11319
Thevaranjan, N., Puchta, A., Schulz, C., Naidoo, A., Szamosi, J. C., Verschoor, C. P., ... & Bowdish, D. M. E. (2017). Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host & Microbe, 21(4), 455–466.e4. https://doi.org/10.1016/j.chom.2017.03.002
Biagi, E., Franceschi, C., Rampelli, S., Severgnini, M., Ostan, R., Turroni, S., ... & Brigidi, P. (2016). Gut microbiota and extreme longevity. Current Biology, 26(11), 1480–1485. https://doi.org/10.1016/j.cub.2016.04.016
Odamaki, T., Kato, K., Sugahara, H., Hashikura, N., Takahashi, S., Xiao, J. Z., ... & Osawa, R. (2016). Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiology, 16(1), 90. https://doi.org/10.1186/s12866-016-0708-5
Nagpal, R., Neth, B. J., Wang, S., Craft, S., & Yadav, H. (2019). Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer's disease markers in subjects with mild cognitive impairment. EBioMedicine, 47, 529–542. https://doi.org/10.1016/j.ebiom.2019.08.032
Kundu, P., Blacher, E., Elinav, E., & Pettersson, S. (2017). Our gut microbiome: The evolving inner self. Cell, 171(7), 1481–1493. https://doi.org/10.1016/j.cell.2017.11.024
Vaiserman, A., & Koliada, A. (2021). Gut microbiota: A player in aging and a target for anti-aging intervention. Ageing Research Reviews, 70, 101413. https://doi.org/10.1016/j.arr.2021.101413



























The Ethics and Implications of Anti-Ageing Research
As exciting as the prospect of reversing or slowing aging may be, it is important to consider the broader implications of this research. Some key questions to ponder include:
Social impact: How would dramatically extended lifespans affect society, from workforce dynamics to healthcare systems?
Economic considerations: Who would have access to anti-aging therapies? Could this create new forms of inequality?
Environmental concerns: What would be the impact on the planet if human lifespans were significantly extended?
Philosophical questions: How would extended lifespans affect our perception of life, death, and the human experience?
These are complex issues that require careful consideration as we move forward with aging research.
I took this from an article I wrote on ageing.